Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
The ever evolving regulatory environment places heightened emphasis on Patient Safety. Navitas Life Sciences serves as a trusted advisor with its rich legacy of experience and expertise, providing unique and personalised solutions grounded in industry best practices.
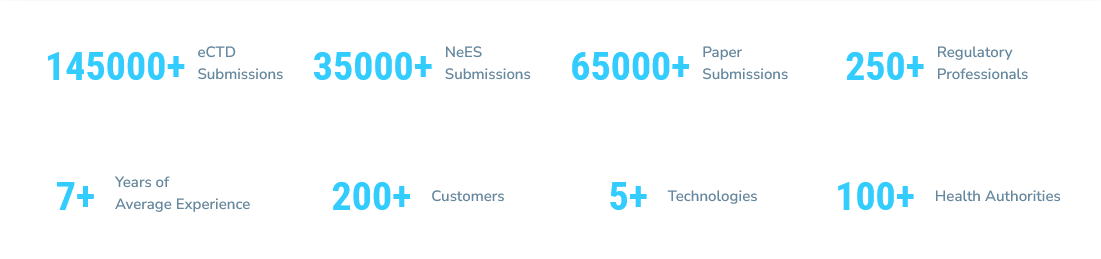
Navitas Life Sciences offers End to End services, supported by a qualified team. Customers can choose specific services or End to End services.
Our End to End regulatory services range from Regulatory strategy, Regulatory CMC, Medical Writing, Labeling & Artwork, Publishing and Submission, Handling Health Authority queries and liaising, Data Management, Regulatory resourcing, and Post marketing support.
Here are 3 recent success stories
1) End-to-End ANDA NCE -1 submission support
A global research organisation based in the US required expert support for ANDA NCE-1 preparation with pre-defined submission window of April 11th, 2021. Navitas Life Sciences initiated preparation and submission in October 2020, the task involved providing regulatory strategy support at all stages of the ANDA, with technical authoring of CMC Documents, Administrative sections, Artwork and Labeling, including provision of an US agent support. Navitas Project Manager coordinated with multiple stakeholders like the API (Active Pharmaceutical Ingredient) & DP (Drug Product) manufacturer, CRO (Clinical Research Organization), Applicant and Analytical labs for the timely submission. The client highly appreciated Navitas Life Sciences’ team support for their ANDA awarded with first to file (FTF) status (Listed in Paragraph IV Patent Certifications (PPIV) in FDA database without RTR (Refuse to Receive). The company also expanded the scope of the engagement in the form of additional dosage strength support and LCM submissions.
Appreciation Received
"We appreciate the team work demonstrated by every team member to meet this major milestone for this project despite the pandemic challenges.
I wanted to register that this relay was supported by all the team members and the final lap was taken up by Navitas Life Sciences publishing and PM team members spending about 140 hrs in the last 7 days.
I sincerely appreciate the dedication and commitment from all the team members."
Head - Regulatory Affairs
2) Large NDA Submission and Publishing support
A pharmaceutical research and development company located in Marlborough, Massachusetts. requested for our support for their Original NDA submission.
The list of services that were requested for included
Navitas team used the BPaas model (Business Process as a Service) , where the Publishing and DMS software is hosted in the AWS (Amazon Web Services) and access provided to client for parallel review, with the submission handled on a rolling basis. This ensured a successful and timely submission to the health authority. The sponsor was very happy with the high performance mindset and collaborative approach showcased by Navitas Life Sciences in delivering this huge assignment.

Appreciation Received
“ Thanks to Navitas Life Sciences for all that you made till now to make this submission happen on time”
Senior Director, Regulatory Affairs
3) Large /Complex submission support for USFDA
A large global Pharmaceutical, based on earlier experience working with Navitas Life Sciences, required support for NDA submission filings for the US market. The submission had more than 3000 patient Client Record Forms , 48 clinical studies and a very large CMC package that included 3000+ documents to handle.
Navitas Life Sciences successfully delivered the Major submission as per the submission plan and received accolades from the sponsor for expert handling of the extensive CMC components & vast clinical trial studies.
Appreciation Received
“A very special Thank You to Navitas Life Sciences team, including to all of the Publishers who supported this large and complex submission. This was the largest CMC package ever submitted in a single filing at our company and the Publishing team was outstanding in following a complex linking strategy and working through the large volume with amazing efficiency”
Director, Regulatory Affairs
Navitas Life Sciences provides Fit-for-Purpose, Cost-effective Solutions for small, mid-sized, and large Pharma companies. We know your needs as experts with 20+ years of experience to provide tailored solutions. Since 2001, our proprietary networks have brought together 60+ leaders in Regulatory Affairs to network and share ideas on how to tackle the latest strategic challenges. Our networks give us benchmark insights into the latest industry trends and enable us to future-proof your strategy and operations.
To know more about our services and solutions, reach out to us at