Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
Navitas Life Sciences has the expertise and experience to support clinical research, partnering with many small to large pharmaceuticals, to change the trajectory of medical science. The deep domain expertise gained by supporting market reach and beyond of drug products has established Navitas Life Sciences as a significant player in the clinical research industry. There are multiple knowledge sharing sessions that are hosted by experts to help pharmaceutical companies react quickly to the changes currently affecting the industry.

Navitas Life Sciences provides pharma companies with a catalyst for change and an opportunity to gain new insights about changing trends. It delivers the opportunity and agility to adapt and grow.
Here are a few top webinars and paper presentations that we have delivered during the first half of 2021.
1) Webinar on IDMP Compliance: Navitas Life Sciences together with CorrIT recently conducted a webinar titled “IDMP is Becoming Mandatory – A Roadmap to Compliance”. The Live webinar explored the deadlines associated with IDMP compliance, opportunities for Enhancing Regulatory Information Management, a global approach to Regulatory compliance, and the technology that supports RIM and Harmonization of Global/Local Term Stores.
The webinar was hosted by Raj Srinivasan, Executive Business Partner, Safety & Regulatory Technology (EMEA & APAC), Navitas Life Sciences and Gary Wilson, Director, CorrIT Limited.
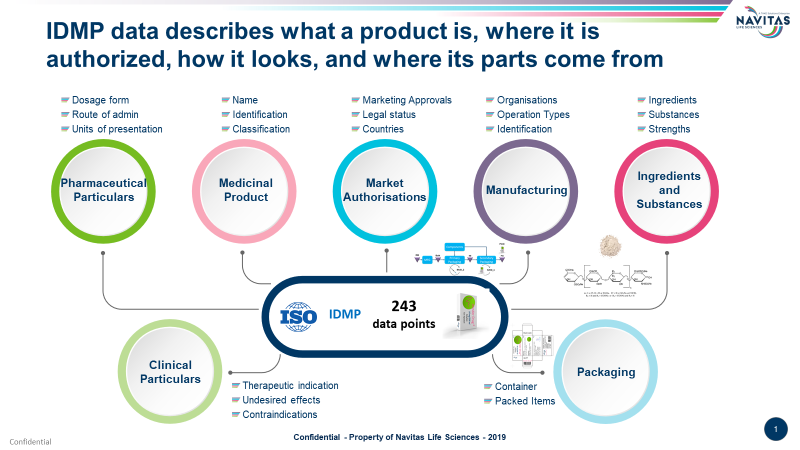
The successful webinar attracted participation from multiple pharmaceutical companies across the globeTo listen to the on-demand version please click here
2) Knowledge sharing On Real World Evidence at the PHUSE India Summer Single Day Event: Navitas Data Sciences presented at the PHUSE India Summer Single Day Event on 12th June, 2021. Navitas Data Sciences is the Functional Service Provider (FSP) of Data Services for Navitas Life Sciences. Our subject matter expert and Principal SAS Programmer, Sasidhar Amilineni, presented a session titled “Real World Data/Real World Evidence – Introduction, Challenges, and Best Practices from a Programmer’s Perspective”
The session discussed more about Real-world data (RWD), which is the data collected from sources like Electronic health records (EHRs), Claims and billing activities, health status from mobiles and wearables, and product and disease registries. Real world evidence (RWE) is the clinical evidence of a medicinal product’s usage, potential benefits, or risks that is determined by analyzing RWD.

The slides for this insightful session can be accessed here.
3) Webinar on how Enabling Digital Quality Transformation at Natco Pharma – Transforming quality operations to achieve robust compliance and high efficiency Navitas Life Sciences and Sparta Systems, a Honeywell Company, have been working together for over 10 years helping to simplify the technology landscape to enable better outcomes for our clients and to drive informed decisions.
We hosted a webinar, together with our client Natco Pharma and our partner Sparta Systems, a Honeywell Company on 15 April, 2021. This was delivered by Raj Srinivasan, Executive Business Partner – Safety and Regulatory Technology at Navitas Life Sciences, Santhosh Francis, Area VP India, ASEAN, ANZ, at Sparta Systems, a Honeywell Company, and Sridhar Reddy, VP Quality, at Natco Pharma. This webinar explored the key industry trends and operational challenges impacting the Pharmaceutical industry, best practices for building a successful QMS business case and ensuring project success, and how Natco Pharma transformed their quality management operations by implementing TrackWise QMS.

4) Webinar exploring Drug Data Coding Conventions – the coding process and challenges: Our Clinical Data Co-orindator II, Imtiayaz Khan, presented at the Uppsala Monitoring Centre WHODrug Webinar on Drug Coding and Data Conventions. His presentation explored data coding processes, as well as their associated challenges addressing the following:
If you would like to view the presentation please click here
5) Knowledge Sharing Session on NextGen PV - A Digitalized Future: Navitas Life Sciences subject matter experts, Louise Tan, Senior Consultant, and Ben Parsons, Managing Consultant, addressed the audience at the Oracle Health Sciences Connect North America on 24 March 2021 with a presentation on the topic of NextGen PV - A Digitalized Future. The session explored how PV of today is facing multiple, evolving challenges with increasing workload, costs and complexity, amidst a data explosion. There were also key insights provided on the PV trends of tomorrow being digital and predictive. To explore this session in more detail please click here
6) Knowledge Sharing Session on Clinical Data Standards: Navitas Data Sciences presented at the PHUSE India Spring Single Day Event on 20th March 2021. Our expert, Shrishaila Patil, Vice President of Statistical Programming, presented a session titled “Clinical Data Standards in the era of Artificial Intelligence, Machine Learning, and Digital Transformation”. The session explored COVID-19 and the wave of Digital Transformation. Why there was a need for Data Standards and the road ahead. The slides from this session are available here
7) Webinar on current and forthcoming ADaM publications: Navitas Data Sciences presented at the CDISC webinar on 2nd March 2021 on ‘Current and Forthcoming ADaM Publications’. Our expert, Luke Reinbolt, Lead Consultant and Clinical SAS Programmer Analyst presented on ADNCA.
ADNCA is the dataset used for Non-Compartmental Analysis, NCA. This is where the Pharmacokinetics (PK) parameters are created by the scientists. Parameter creation utilizes software with certain dataset requirements and existing BDS and/or SDTM structures do not meet requirements. Prior to ADNCA, it meant submissions contained interim datasets configured as needed for the software without guidance as to structure; thereby resulting in many different dataset structures. ADNCA provides such guidance and standardizes this part of the PK process. ADNCA has completed the public review.
Navitas Data Sciences provides end-to-end solutions

8) Knowledge Sharing Session on Electronic Product Information: At the DIA Regulatory Submissions, Information, and Document Management Forum that took place on 10 February, our subject matter expert, Tris Nockles presented as part of Track 1: IRISS Forum Hot Topics. Her session explored the Electronic Product Information.
At Navitas Life Sciences we use awareness and education to engage all stakeholders to help cope with the evolving life sciences sector. Participate in our webinars and stay abreast with the latest insights from the pharma world.
Topic: Shifting Sands of Pharmacovigilance - Pivot, Maneuver, Breakout
Date: 11 August, 2021
Time: 11:00 – 12:00 EST | 17:00 – 18:00 CEST | 20:30 – 21:30 IST
Join our subject matter expert, Dr. Latika Sharma, PV Networks Lead at Navitas Life Sciences as she provides expert insights on the future course of pharmacovigilance addressing the following key themes: