Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
There is an increasing focus on diversity in clinical trials, which is crucial for providing essential data on the safety and efficacy of new treatments before they are approved for widespread use. However, a significant issue plaguing the clinical trial process is the underrepresentation of older adults. This lack of age diversity in clinical trials can have serious implications for patient care, the validity of results, and the overall success of medical research.
The Problem of Underrepresentation
Older adults are often excluded from clinical trials, resulting in a significant gap in our understanding of how new treatments affect this demographic. This exclusion is problematic because older individuals are typically the most frequent users of medications, yet the safety and efficacy of these drugs in the elderly population remain largely untested. Consequently, physicians are left with little information on how to best treat their older patients, raising critical questions about medication safety and effectiveness in this age group.
Historical Context and Ethical Concerns
The exclusion of elderly patients from clinical trials is rooted in a desire to protect vulnerable populations from potential harm. Historical instances, such as the thalidomide disaster of the 1950s-60s, highlight the severe consequences of insufficiently tested medications on specific groups, such as pregnant women. This incident underscores the ethical dilemma researchers face: the need to gather comprehensive data versus the imperative to protect participants from harm.
However, this cautious approach has led to a significant underrepresentation of the elderly in clinical trials. The reluctance to include older adults stems from concerns about their increased susceptibility to adverse effects and the presence of comorbidities that could complicate trial outcomes. Yet, excluding them entirely does a disservice to this population by limiting the applicability of research findings and perpetuating a cycle of inadequate data for clinical decision-making.
Navitas Life Sciences champions age diversity in clinical trials to enhance research, improve outcomes, and ensure fair representation, benefiting all patient populations.
Including a diverse age range in clinical trials offers numerous benefits that enhance both the patient experience and the success of the trial itself. When older adults are included, the results of clinical trials become more generalizable, providing a clearer picture of how treatments will perform in the broader patient population. This inclusivity helps ensure that medications are safe and effective for those who are most likely to need them, thereby improving patient outcomes.
Another interesting aspect is that age diversity in clinical trials can lead to better patient experiences. Older adults bring unique perspectives and needs that can inform the design and conduct of trials, making them more patient centric. This approach aligns with the industry's growing emphasis on patient centricity and trial diversity, which seeks to make clinical research more inclusive and reflective of real-world patient populations.
FDA guidance on diversity in clinical trials has been instrumental in addressing these challenges. The FDA diversity in clinical trials guidance aims to improve the inclusion of various demographic groups, including the elderly, to ensure that clinical trial data accurately reflects the populations that will use the treatments. The FDA diversity in clinical trials 2023 guidelines emphasize the need for age diversity, highlighting the importance of including older adults in research.
Increasing diversity in clinical trials involves several strategies. First, researchers should reconsider age limits and evaluate eligibility on a subject-by-subject basis, avoiding an all-or-none mentality. Actively recruiting older subjects and educating them about clinical research can also help reverse trends of underrepresentation. Learning from efforts to recruit marginalized populations and engaging patient advocacy groups can further enhance age diversity in trials.
The Role of Regulatory Bodies and Industry Leaders
Regulatory bodies like the FDA have a crucial role in promoting age diversity in clinical trials. The FDA guidance on diversity in clinical trials sets out requirements for including older adults in research, recognizing that a representative sample is vital for generalizable and applicable results. For instance, the guidelines recommends a minimum of 100 geriatric patients in drug trials for diseases prevalent in older populations, to detect significant differences in drug responses between age groups. The Robert A. Winn Diversity in Clinical Trials initiative aims to address and overcome barriers to inclusion, ensuring a more representative and equitable patient population.
Despite these regulations and support, older adults are still under represented in clinical trials. In oncology, for example, while 42% of patients are over the age of 70, less than 10% of clinical trial participants are from this age group in National Cancer Institute sponsored clinical trials. This gap highlights the need for more robust efforts to recruit and retain elderly participants in clinical research.
Practical Steps for Enhancing Age Diversity

With FDA diversity in clinical trials guidance and initiatives like Robert Winn Diversity in Clinical Trials, the industry is moving towards more inclusive research practices. By simplifying participation, providing humanized support, and ensuring transparency, we can address the lack of diversity in clinical trials and improve the overall success of medical research.
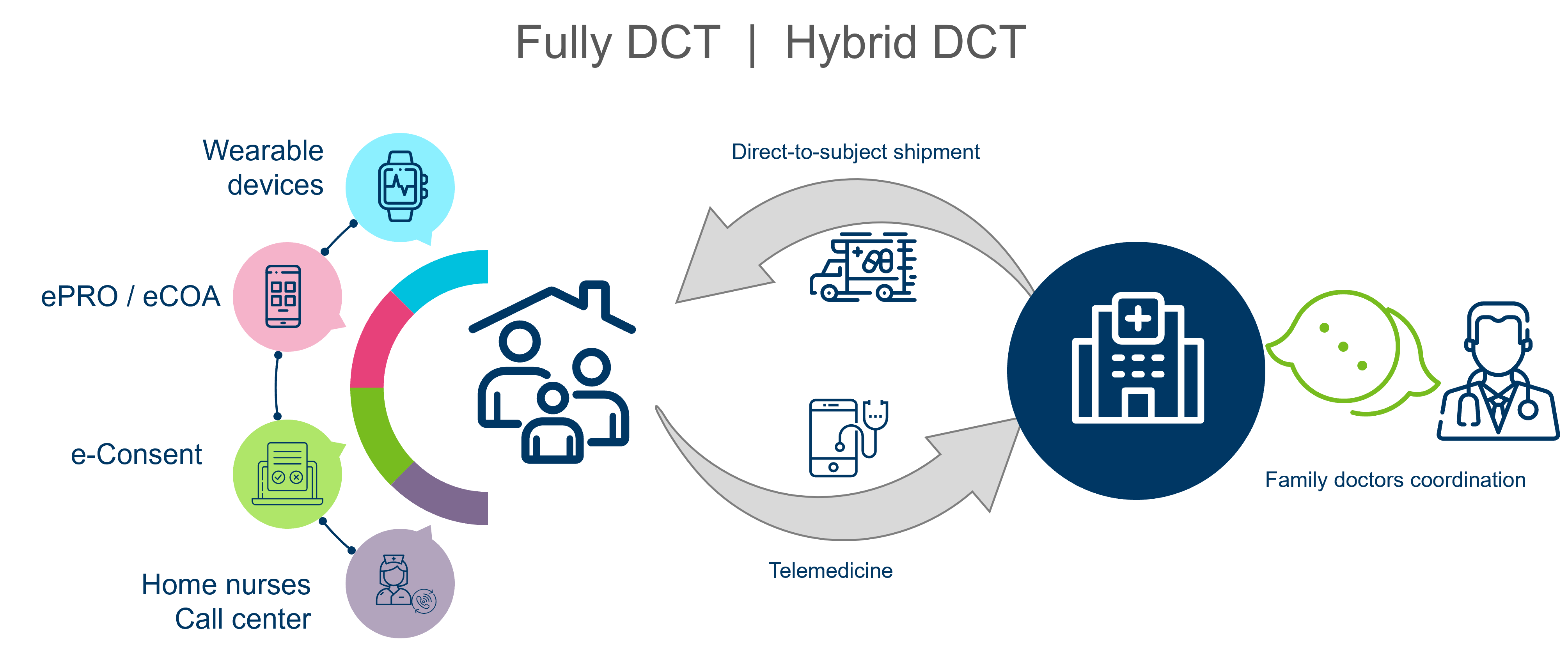
Decentralized clinical trials (DCTs), which use technology to conduct research outside traditional clinical settings, offer a promising avenue for increasing age diversity. DCTs can reduce geographical barriers and facilitate participation from a broader socioeconomic range. However, the reliance on digital tools in DCTs can be a challenge for older adults. Therefore, efforts must be made to ensure that technology is user-friendly and that participants have access to necessary support.
Regulatory Changes and Industry Practices
Regulatory changes can drive significant improvements in age diversity. For example, the FDA's requirement for including a minimum number of geriatric participants is a step in the right direction, but more can be done.
In addition to regulatory efforts, the industry must also take proactive steps. Simplifying patient participation, providing clear and transparent information, and offering robust support systems are all critical measures. These efforts not only improve the patient experience but also ensure that clinical trial data is more reflective of the general population, leading to better-informed medical decisions.
Navitas Life Sciences Support Diversity in Clinical Trials
Age diversity in clinical trials is essential for ensuring that medical research is truly representative of the populations it aims to serve. By including older adults in clinical trials, we can enhance the generalizability of research findings, improve patient outcomes, and promote fairness and trust in the medical community.

At Navitas Life Sciences, we leverage our vast and diverse patient population to manage efficient and representative clinical trials. We are committed to supporting this goal by working with industry stakeholders to actively promote the inclusion of older adults in clinical research. Through these efforts, we can fully realize the potential of clinical trials to benefit all patients, regardless of age.
To ensure successful outcomes for your clinical trial, partner with a team that understands the objectives of the study, enables data-led decision making, and provides trial oversight & incisive insights to keep the study on track to meet timelines and deliverables.
Navitas Life Sciences ensures adherence to study timelines and milestones through:
All of our clinical trials services are powered by OneClinical® analytics, our source system, format agnostic, data driven, and ICH E6 R(2) compliant eClinical platform.
To learn more about our services and solutions, reach out to us at
References