Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
International Clinical Trials Day is an annual event celebrated on 20th May 2023 to acknowledge the pivotal role clinical trials play in advancing medical science and improving patient outcomes.
Clinical trials can be traced back to the time when early healers and physicians experimented with various treatments and remedies. However, it was not until the 18th century that clinical trials, as we know them today, began to take shape. British naval surgeon James Lind is often regarded as the pioneer of clinical trials. In 1747, he conducted a groundbreaking study to determine the effectiveness of citrus fruits in combating scurvy among sailors. This work laid the foundation for systematic experimentation and paved the way for future advancements in medical research.
Navitas Life Sciences supports small to large pharma, biotech and devices companies, generics manufacturers, and U.S federal agencies like Centers for Disease Control and Prevention (CDC) and The U.S. Department of Defense (DoD), delivering platform-driven full-service Clinical, Regulatory and Safety solutions and services.
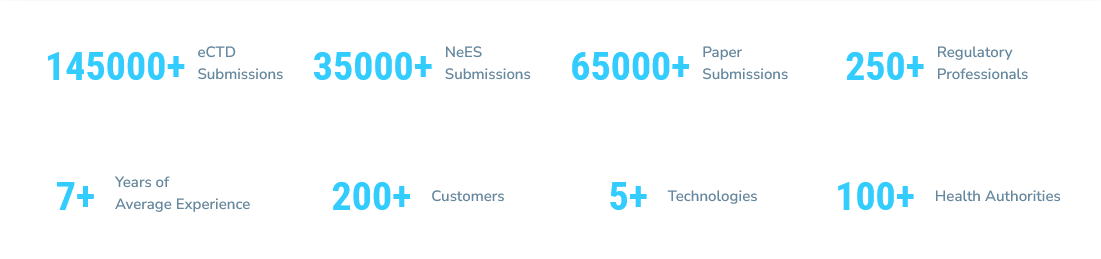
A technology led full-service CRO, Navitas Life Sciences has 30+ years of rich experience across 600+ phase I-IV clinical trials, 20+ therapeutic areas including oncology. Our trial expertise is augmented by our proprietary OneClinical® Analytics, a platform that delivers trial oversight and key data insights that aid in better decision making for efficient clinical trial management.
Clinical Trials have a wide value base and enrich medical science and the patient community at large. They have multiple benefits including:
1) Advancing Medical Knowledge while leveraging the latest methods and technology:
Clinical trials have emerged as the gold standard for evaluating the safety and efficacy of medical interventions, ranging from new drugs and therapies to medical devices and preventive strategies. They serve as a essential bridge between laboratory research and real-world patient care. By subjecting investigational treatments to rigorous testing in diverse populations, clinical trials generate robust evidence that guides medical decisions, informs regulatory approvals, and ultimately enhances patient care.

We build bespoke teams of highly skilled Statistical Programmers (both US-based and Global Programmers), Biostatisticians, and Data Managers. Our expert-driven Statistical Programming, Biostatistics, and Data Management services support our sponsors in reaching successful clinical trial outcomes. Our blended team of highly skilled experts fuel every FSP engagement by providing complete data support or by augmenting existing teams.

2) Improving Patient Outcomes and Delivering Support for Better Care:
Clinical trials have played a transformative role in the prevention, diagnosis, and treatment of diseases. They have been instrumental in the development of life-saving drugs, breakthrough therapies, and innovative medical devices. From the discovery of antibiotics and vaccines to the advancements in precision medicine and gene therapies, clinical trials have revolutionized healthcare by extending life expectancy, improving quality of life, and providing hope to millions of patients worldwide.
Navitas Life Sciences has been supporting clinical trials across 20+ therapeutic areas, including oncology.

3) Accelerating Drug Development and faster Market Reach:
In addition to their impact on patient care, clinical trials are vital for the pharmaceutical industry and regulatory agencies. These trials help pharmaceutical companies gather essential data required for regulatory approvals and commercialization. By fostering innovation and expediting drug development, clinical trials contribute to the timely availability of safe and effective treatments, addressing unmet medical needs and combating global health challenges.
Navitas Life Sciences leverages its vast experience, expertise and technologicl support to drive innovation in clinical trials, resulting in cost and time efficient clinical trials.
Regulatory support and pharmacovigilance play a vital role in ensuring safety of drugs and medical devices. Let us look at their significance and importance on International Clinical Trials Day:
With a rich legacy of experience and expertise, Navitas Life Sciences serve as trusted advisors and provide tailored End-to-End Regulatory solutions grounded in industry best practices
Pharmacovigilance and Safety Monitoring: Pharmacovigilance, the science of monitoring and evaluating the safety and effectiveness of medical products, is of paramount importance. Regulatory agencies oversee pharmacovigilance efforts to detect and manage adverse events associated with investigational treatments. Robust pharmacovigilance systems ensure that any potential risks or side effects are promptly identified, reported, and appropriately addressed, enhancing participant safety and the reliability of trial results.

Navitas Life Sciences has been at the forefront of Pharmacovigilance for 30+ Years. We understand the crucial need for Life Sciences organizations to leverage technology to automate processes, use data to drive insights and hence, improve efficiencies while supporting the safety profile of their drugs.

Joining one of our industry leading networks can help raise the profile of the company and the team. The networks provide a chance to meet peers and be part of an independent sounding board to test and validate the latest thinking, and uncover industry insight to develop compelling, yet pragmatic strategies to provide business value and meet the needs of patients, payers, and prescribers.
Regulatory support and pharmacovigilance are indispensable components that ensure patient safety, maintain ethical standards, streamline trial processes, and monitor safety and efficacy.
International Clinical Trials Day recognizes and appreciates the remarkable contributions of clinical trials to medical science and patient care. As we celebrate this day, let us renew our commitment to supporting and advancing clinical research, with the shared goal of achieving better health outcomes for people in need.
Date: 23 – 24 May 2023
Venue: Valley Forge Casino Resort, King of Prussia, PA, USA
Exhibiting at: Booth 49
Navitas Life Sciences is delighted to showcase as Technology Spotlight Sponsors at the upcoming Outsourcing in Clinical Trials East Coast 2023.
To Know more about our services and solutions, reach out to us at