Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
Celebrating 200+ pharmaREADY® customers and 100% satisfied users

Marty Boom,
Global Head of Regulatory and Safety,
Navitas Life Sciences
“The pharmaREADY® technology, combined with a team of experts who are truly committed to enhancing the experience of our clients; with hands-on support and sharing of knowledge of regulations and best practices, makes a highly attractive and compelling proposition. We are proud to be able to offer this proposition to 200+ clients across the globe.”
Regulatory Affairs play a pivotal role in bringing new drug products to the market swiftly. However, it is often perceived as comprising a heavy manual workload that requires repetitive human input. Regulatory technology solutions support the need to reduce manual processes, while focusing on the pursuit of operational excellence.
According to a report by Businesswire, the global regulatory technology market is expected to rise at an estimated 19.5% CAGR during the period 2020-2021 to 2026. The growth is expected to be fueled by an increase in demand for risk management, as well as a growing requirement for regulatory intelligence and monitoring solutions.
Strategically partnering with our clients, we are driving accelerated compliance and delivering first-time-right submissions. Navitas Life Sciences’ pharmaREADY® is a fully integrated, regulatory compliant, web-based suite comprised of Document Management, Training Records Management, Structured Product Labeling, and eCTD Publishing Solutions. pharmaREADY® simplifies the complex process of creating, viewing, and managing submissions – electronic, NeeS or paper.

pharmaREADY® has the added advantage of being low cost (lowest total cost of ownership in the industry) and available via the cloud or as an on-premise regulatory suite. Intuitive and easy to use, with overall implementation in 3 to 4 weeks.
The main purpose of regulatory affairs is to ensure that drugs, that are more effective, and which are safer, are provided the necessary approval. pharmaREADY® is an innovative solution to contain the pressure on regulatory submissions and to improve the productivity of the team.

During implementation, our team of expert navigators will provide training and walk you through your first submission; ongoing 24/7 technical support is also provided. It is compliant with 21 CFR Part 11, Annex 11, cGMP, and HIPAA ensuring that companies will always be inspection ready.
Simple data migration utilities ensure easy migration of legacy data including documents, submissions, and labels in a batch, with no user intervention required, saving time. pharmaREADY® allows old methods to be used, especially to correct mistakes while performing Life Cycle Management (LCM) on imported legacy submissions.
Launched in 2005, pharmaREADY® has evolved over the past 15+ years to become an intuitively designed and robust product suite that we have implemented across the globe for 200+ Life Sciences companies. There are multiple modules to choose from, to suit your unique needs
A Collaborative Workflow to manage your documents throughout their life cycle. Teams can create, review, approve, distribute, and archive documents with automated version control, audit trails and electronic signatures.
Publishing of your Regulatory Submissions through eCTD/NeES/Paper formats to comply with Regulatory Health Authority requirements.
SPL Conversion of Labeling content to comply with US FDA eLabeling requirement along with Physician Labeling Rule (PLR).
Manage and Archive training documentation for all business areas as per global Regulatory Health Authority requirements to ensure inspection readiness and compliance.
A mid-sized pharmaceutical company headquartered in the UK, researching and developing novel dermatological treatments wanted to go fully paperless, including their audits. This would allow their team to remotely review and approve documents. Supported by a progressive IT department, they wanted the solution to work with the latest Microsoft technologies to ensure their technology stack would remain current.
The footprint of the company was expanding, and they needed to prepare for First-Time-Right electronic submissions with electronic signatures as per 21 CFR Part 11 to global Health Authorities (HAs) and obtain faster marketing approvals. In addition, they wanted to be able to migrate legacy submissions for further Life Cycle Management submissions.
Our client required the eCTD publishing setup as per the HA mandate and be as automated as possible to reduce the overall time taken, without affecting the quality of the output.

To read the case study in full, please click here
Strategic collaborations are becoming increasingly important in the life sciences sector to strive for greater efficiency, insight and to drive better productivity. In order to do this, it is important for seamless collaborations that allow easy implementation and effective support systems.
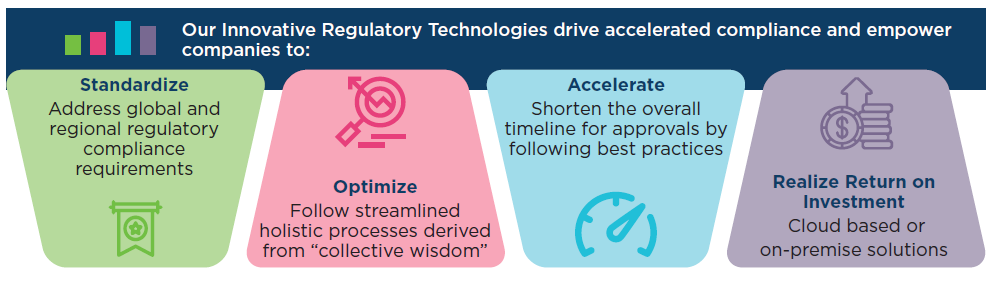
pharmaREADY® has been developed to ensure a robust process optimization for First-Time-Right submissions, while also recognizing the need for our technology to be both adaptive and flexible to support an ever-evolving regulatory environment across the product life cycle.
To learn more about pharmaREADY®, click here, or reach out to us at