Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
Technology has revolutionized the healthcare continuum, altering patient safety worldwide, while lowering costs and time. Recent reports have estimated that the pharmacovigilance (PV) and drug safety software market valued at USD 163.08 million in 2020 is estimated to touch nearly USD 240 million in 2026, with the CAGR at 6%.
An increase in incidence of adverse drug reactions (ADRs) is expected to lead to a growth in the PV and drug safety software market. According to a study published in 2019 by the U.S. Centers for Disease Control and Prevention (CDC), each year nearly 700,000 emergency visits and 100,000 hospitalizations occur due to adverse drug events (ADE).
With approximately 5% of patients hospitalized experiencing an ADE, there is a need for scientifically robust technology solutions for improved patient safety.
It is imperative to monitor, as well as to track, the accuracy and efficiency of a drug, ensuring that safety issues are entered and investigated when they arise. This will avoid delays and inaccurate reporting that could attract fines, time delays, and lack of regulatory compliance.
Moreover, life sciences companies face challenges in trying to stay updated with evolving regulatory compliance requirements. Therefore, it is important to keep the pace at which the regulatory authorities are updating compliance requirements.
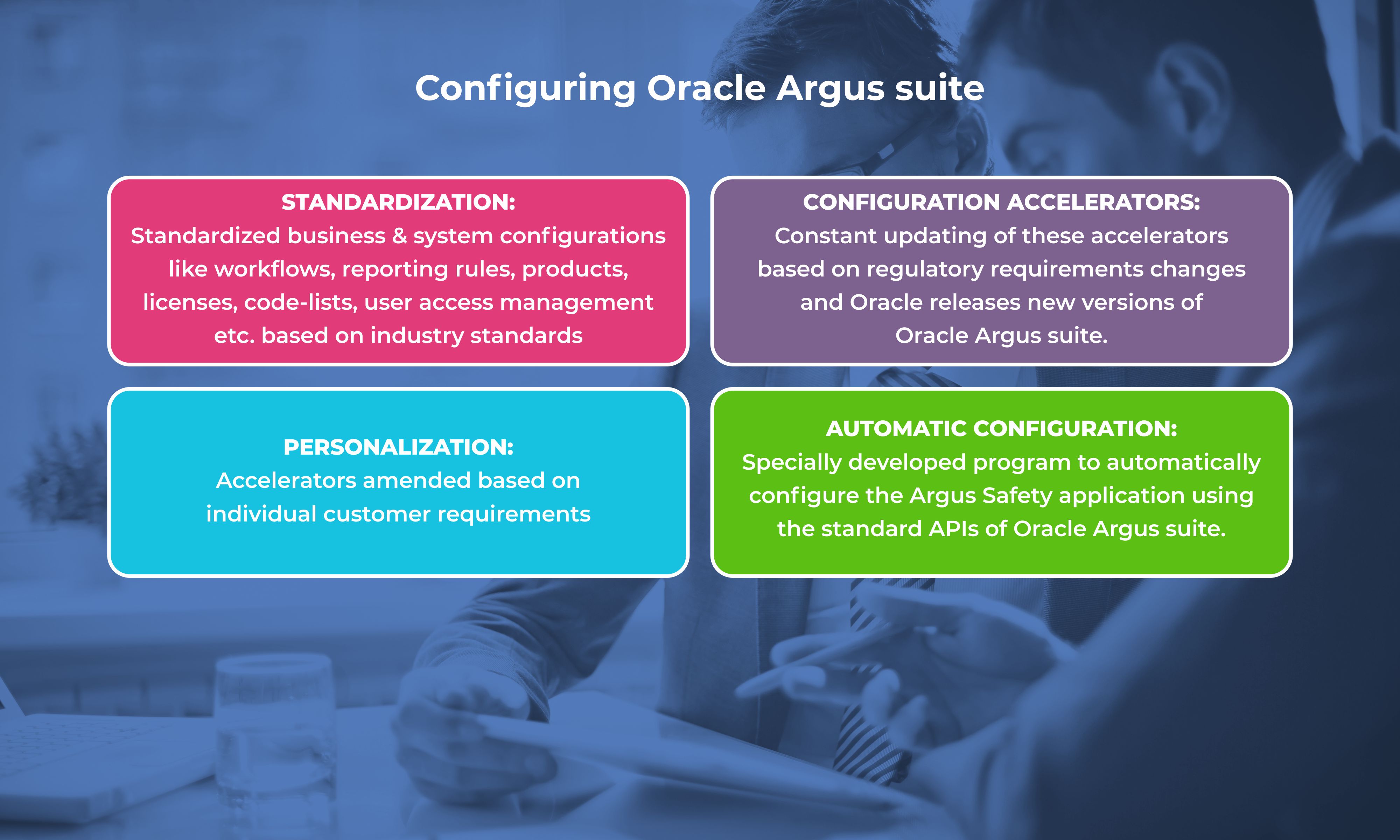
Oracle Argus improves global compliance, increases efficiency of safety operations, and enhances case quality for faster and more informed decisions, but it is a challenge to implement it at optimal cost and within timelines.
“I would particularly like to commend the Navitas Life Sciences project leader for his thoughtful, quiet leadership that somehow brought all the right people together and led to a true understanding across disciplines. Also, project members, for their very high level of expertise, excellent communications, and skill in leading the team to implement client requirements so smoothly.”
Dr. Peg Fletcher,
President, MedAssessment, Inc.
Implemented Argus Safety with Navitas Life Sciences
Navitas Life Sciences has created safetyREADY® to address such challenges. Oracle Argus is now available in the multi-tenant cloud and can be deployed rapidly using a pre-configured and pre-validated environment.

We met Mr. Subash J, Senior Program Manager, Delivery-Safety, at Navitas Life Sciences to gain insights about safetyREADY® and how it will help in improving pharmacovigilance systems.
Tell us a bit about your professional background
I have over 13+ years of experience managing multiple engagements within Life Sciences and I have handled a number of Oracle Argus implementations, upgrades, data migration projects, as well asintegrations. I have been instrumental in developing new solutions specific to Pharmacovigilance/Drug Safety and have built accelerators to fast-track implementation.
Please give us a glimpse of your role at Navitas Life Sciences
I manage the Safety Technology Delivery practice at Navitas Life Sciences, ensuring that there is a seamless transition while also providing excellence in service support.

Mr. Subash J,
Senior Program Manager,
Delivery-Safety,
Navitas Life Sciences
How does safetyREADY® bridge gaps in support for Pharmacovigilance?
safetyREADY® aids in accelerating the implementation and validation of the Oracle Argus suite of applications with utmost quality and commitment. “The perfect amalgamation of technology accelerators and global safety regulations (21CFR Part 11, EU Annex 11 requirement) and GAMP guidelines.”
What makes safetyREADY® ideal for Oracle Argus Implementation?
safetyREADY® is compliant to global safety regulations, with shorter delivery cycles, low operational cost, scalable infrastructure, and provides real time insights to inform decisions.
How difficult is it to migrate to safetyREADY® Cloud?
We utilize our safetyREADY® Migrate utility feature for efficient and quality migrations of safety data. A benefit of safetyREADY® migrate is that it is both source and target agnostic. We follow a three-level validation approach to reduce the risk of data loss at the time of migration.

Prebuilt migration packages for rapid migrations from legacy applications
What is the training you provide for using safetyREADY®?
We provide focused training to all business users as well as Admin users to ensure that they are informed and comfortable using the application. In addition, we supply training materials and user guides to end users to use as reference guides as and when required. Post go-live, we have experienced support personnel available 24/7 providing world-class application support.
How does safetyREADY® help organizations stay compliant within an ever changing regulatory environment?
As a leading PV consulting organization, Navitas Life Sciences benefits from the latest insights in the PV technology space which we derive from benchmarks and discussions with the membership of our industry leading network pvtech®. In addition, as an Oracle Gold Partnerwe work closely with the Oracle team to best understand the future direction of the Oracle Argus product in terms of how it will address any upcoming changes to regulatory requirements. Based on the inputs, we define the roadmap for our safetyREADY® applications, which helps us to ensure that organizations are able to stay compliant with the ever evolving regulatory changes.
How are client requirements incorporated into solutions?
We value feedback from our customers and use it to evaluate client specific requirements and upgrade our solutions and accelerators accordingly.
How can safetyREADY® be used for audits and regulations?
As an implementation partner, we support our customers during regulatory and internal audits.
How does safetyREADY® cope with the changing digital trends in adverse event reporting?
We have designed a framework that allows the accurate reporting of adverse events using a smart phone or brand website. Navitas Life Sciences has also built a connector between these sources and Oracle Argus Safety, so that the reported data is entered into the Argus system as a validated form.
Gain from decades of PV Consulting experience and several years of successful Argus Safety suite Implementations
To find out more about our service and solutions, please reach out to us at