Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
Several times during a clinical trial, experts work along with sponsors to strategize with the best intentions to assess clinical trial progress and mount a coordinated response towards efficient management. There may be instances when protocol deviation may be necessary to overcome challenges faced during the clinical trial run.
Navitas Life Sciences utilize ICH E6 R2 approach to support protocol deviation (PD). Once a protocol deviation is identified, it needs to be classified into important and non-important. According to the ICH E3 Q&A R1 sponsors can decide what an important PD is, and the determination of the same is based on the study designs, study data, essential procedures as included in the protocol as well as in the study data’s planned analysis.
Periodic Review: It is important to apply critical thinking and to analyze the progress of the clinical trial periodically.
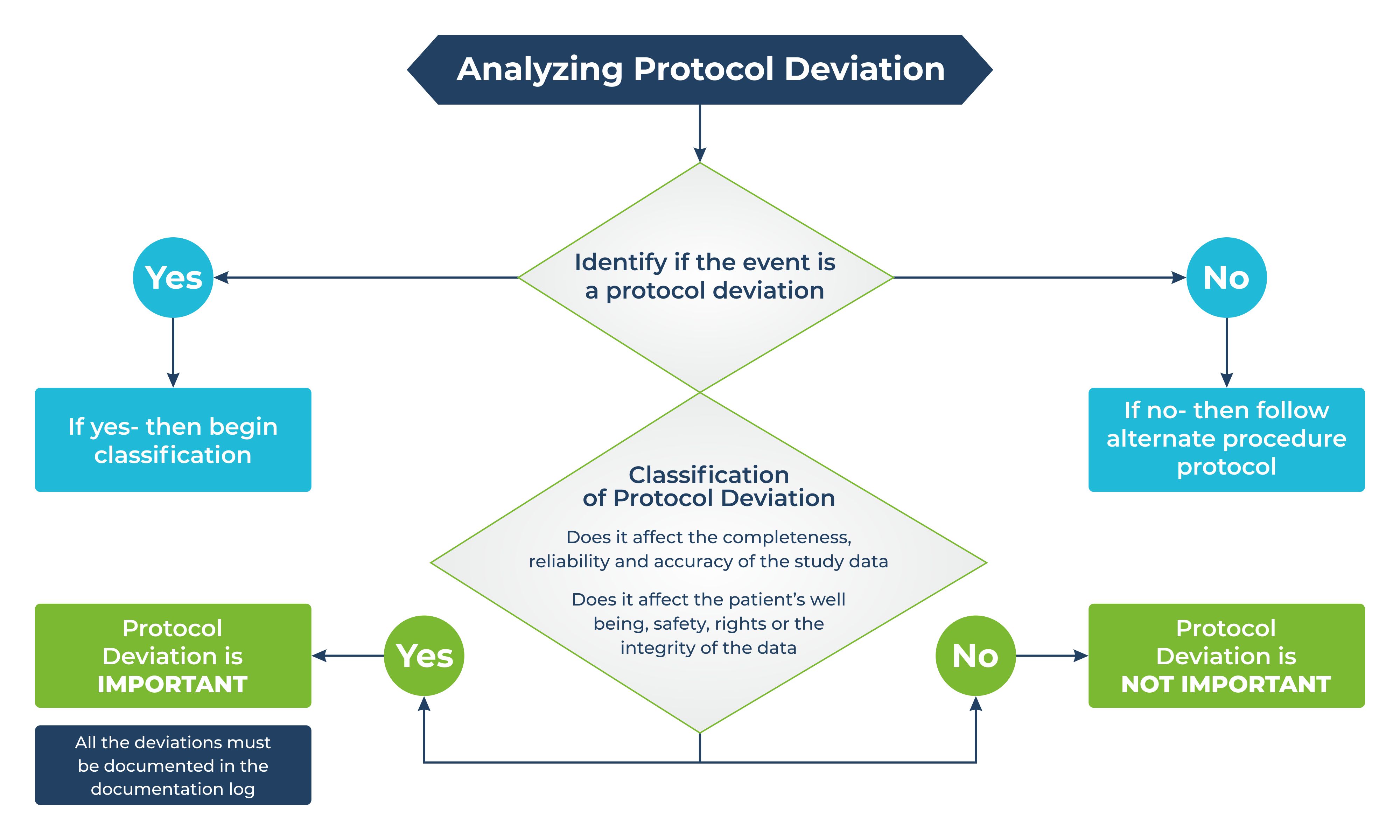
Navitas Life Sciences conducts clinical trials following Good Clinical Practice (GCP). Issues raised during GCP should not be brought under the purview of PD unless it is deemed important. Routine escalation procedures are followed for issues raised based on GCP.
The protocol is the referenced document to determine if an event can be listed under protocol deviation. It is imperative, therefore, to design the protocol after carrying out a risk assessment review and to define protocol deviation, even before finalizing the protocol.
Such proactive approaches will help in reducing the number of events listed under PD during the study.
The first step in defining a protocol is to identify important PDs that could take place. The information is gathered from an organizational, study based and protocol level components. Organizational level information includes institutional policies, regulations and SOPs. Study based aspects include drug compounds, therapy area or study indications. Protocol level components include issues to further enhance the definition. This brings about consistency while reporting incidences.
All the study members are trainer suitably to follow the protocol and to identify and report any deviations that may occur.
Protocol deviation may be identified using manual procedure or using digital system. In a digital system, protocol deviation may be identified based on the data entered into the system while manual procedure depends on human reporting system.
Both methods should be utilized for identifying protocol deviations. Clinical research associates are trained to identify deviations that are not captured by the digital systems.
A risk-based method of identifying protocol deviation will help in identifying important PDs. It will help in identifying PDs that could potentially impact patient safety or affect the study data.
All PDs in a study are stored and are analyzed during periodic review meetings. The important PDs are reviewed based on ICH E3 guidelines. All stakeholders are apprised about the PDs that occurred with identification of potential safety concerns, if any.
Different types of PDs should be identified, analyzed and aggregated based on the risks involved to patient safety and clinical trial data. The identification of PDs will also help in improving the value and quality of clinical trials conducted.
Clarity in purpose and communication is important to support a holistic approach to protocol deviations. Navitas Life Sciences utilizes its experience and expertise to follow a consistent method of PD identification, classification, analysis and reporting.
To know more about our services and solutions, reach out to us at