Delivering Full QPPV Services with a Global Network of Local Experts
A Qualified Person for Pharmacovigilance (QPPV) is personally responsible, by law, for the safety of the human pharmaceutical products marketed in the EU. The QPPV must reside in the EU and provide global support through a network of in-house and local QPPVs.
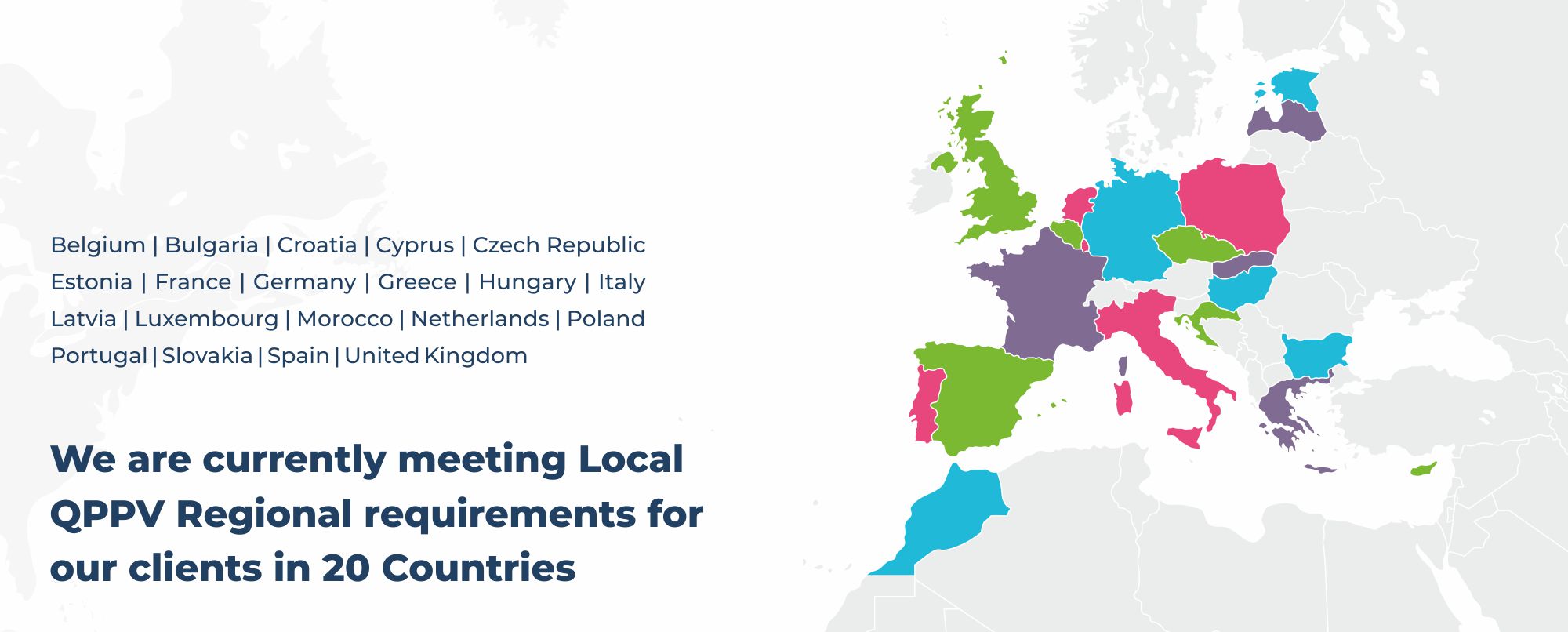
When selecting a QPPV partner, it is important to choose one with access to top local experts who can offer scalable and customized solutions tailored to specific needs. At Navitas Life Sciences, we have a well-established network of local QPPVs who assist our clients in meeting local PV requirements. Our dedicated team delivers integrated, end-to-end solutions that extend beyond QPPV services to include comprehensive safety reporting, clear communications, and effective stakeholder support.
Essential Role of the QPPV
Focus on PV
Safety First
Follow Legislation
Sole Contact
Audits and Inspections

Our Full QPPV Services include:
- Availability of QPPV and local QPPVs
Providing appropriate back-up for the EU and the UK as required by local Health Authority (HA) regulations - 24/7 Point of Contact for Competent Authorities
Including back-up QPPVs for the EU and locally as needed - Preparation, Maintenance, and Review of PSMF
Ensuring the Pharmacovigilance System Master File (PSMF) is always up to date and compliant - Ongoing monitoring or local safety parameters
Including the preparation of aggregate reports, signal detection, and assessment of benefits and risks of MAH products
Our Tailormade Solutions Can Cater for Your Unique Needs
A Marketing Authorization Holder can outsource the function of the QPPV as per need. This could range from a few hours per month and additional hours of QPPV consulting to a full QPPV office with a QPPV, deputy QPPV, and administrational support. At Navitas Life Sciences our solutions are based on your portfolio and geography, with customizable solutions based on your specific needs.
The setting up of a QPPV Office, nominations to Competent Authorities, and related documentation

This model includes a set of routinely performed activities by our QPPVs on a monthly basis


Such activities may be out of scope, but can be executed with client consent
We appreciate that additional and ad-hoc activities arise, and we are happy to support your requirements when they do. Such work is invoiced on an hourly basis as needed. In our experience, such activities may include, but are not limited to:
- Registration CA as MAH’s EU-QPPV and/or Local QPPV
- Develop/adapt/update/inputs/consultation of/to MAHs PSMF (or local) and its sub files and applicable SOPs
- Participation in MAH training, teleconferences, face-to-face meetings, and handling of CA/MAH/HCP requests
- Participate and/or deliver Project specific training to customer/distributors
- Product quality complaints management (translations: to/from English, QC, form filling, QC of filled information, duplicate check and tracking, reporting to CA or Client)
- Processing of identified literature safety information (translation, filling form, sending to MAH), including follow-up activities
- Local RMP related activities and/or risk minimization implementation activities
- Submission of foreign safety reports to CA
- Collection and exchange of CA questions/requests and replying to local medical enquiries
- Participation in audits/inspections, CAPA/deviation management
- Patient Support/Patient Oriented/PASS/PAES activities - protocol design review, tracking
ARTICLE
Automation of literature search and review
ARTICLE
The crucial role of PV in combatting counterfeit drugs
ARTICLE
Current status of biosimilars and their impact on PV
Gain the Navitas Life Sciences Edge
Right Expertise with optimal infrastructure