Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
Navitas Life Sciences has been supporting efficient clinical trials for over 30 years to bring life-saving drugs to the market sooner. Patient centricity has been at the heart of our clinical trials, as it is important to align with the needs and wants of patients to improve patient engagement during the trial.
According to a study, 86% of clinical trials fail to meet deadlines, with patients joining the study being one of the main causes. At Navitas Life Sciences, we adhere to all trial timelines and have a large volunteer base, with patient centricity being an important focus area. We have over 140,000 patients to support our study needs.
Consistently evaluating patients' opinions, viewpoints, and experiences in clinical trials yields valuable insights. At Navitas Life Sciences, our vast patient population and volunteer database is possible owing to our deep understanding of patient needs.
The insights gained from the patients involved in studies serve as valuable tools to create and execute impactful engagement strategies and techniques. Seamless operation of clinical trials centered around patients is imperative to minimizing unnecessary participation challenges. Solutions that enhance convenience, whether rooted in technology or other approaches, can alleviate several obstacles and demands associated with participation.

Incorporating patient perspectives into clinical trial design results in streamlined endpoints, reduced data collection, and fewer protocol amendments. By aligning protocols with patient needs, the trials showcase improved patient engagement, enhanced trial efficiency, and development of treatments that better serve patients. Decentralization is most important aspect of patient centricity, with aim at reducing the in-hospital visits:
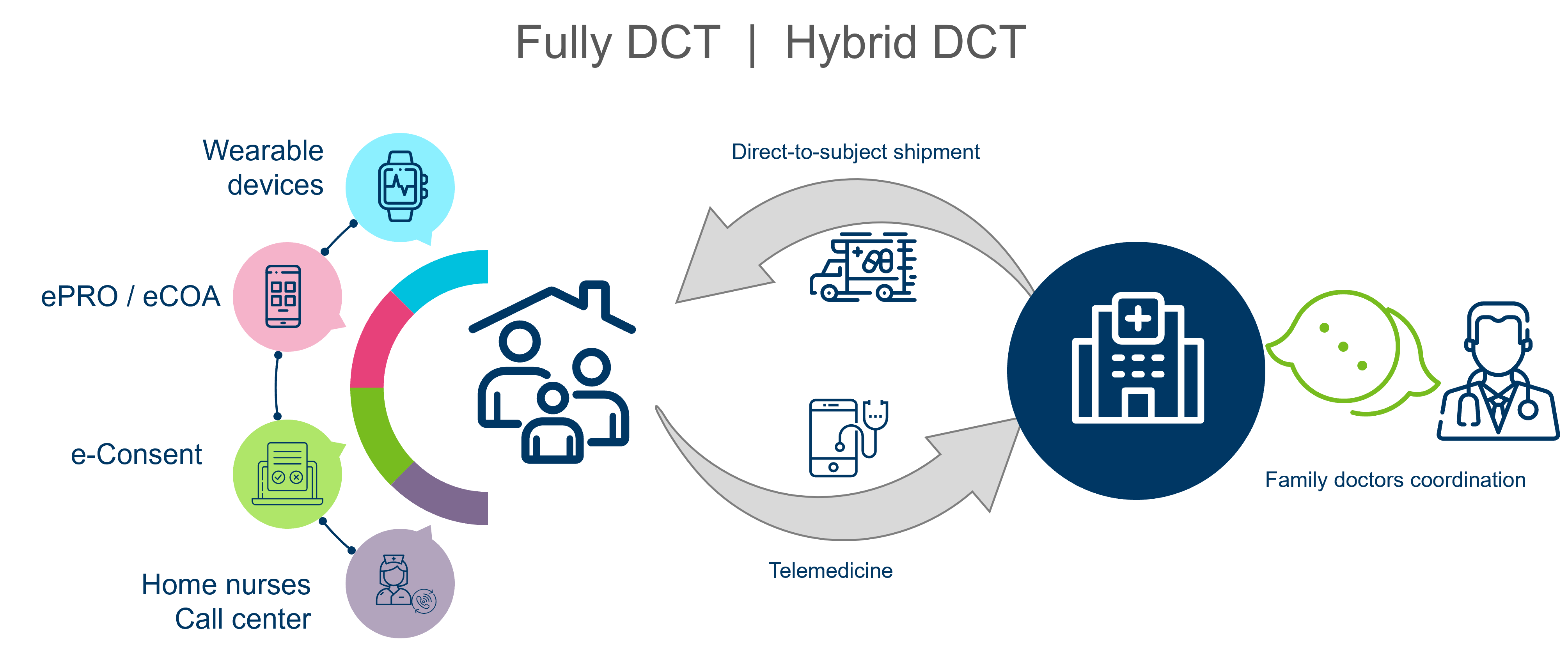


Incorporating patient preferences and unmet needs in clinical trial design allows the development of patient-friendly protocols from the start of the study. This approach reduces the burden on patients, making trial participation more manageable and increasing retention rates. Patients' willingness to participate in trials improves when they perceive that their requirements are understood, reducing discomfort and ultimately leading to trial completions and improved healthcare outcomes.
Recognizing patients' wants and needs and incorporating their perspectives into clinical development programs is a pivotal step toward improving the efficiency and effectiveness of clinical research.
With the rising complexity of clinical trial protocol designs, the engagement, and retention of eligible patient participants throughout the study's duration is imperative to the success of clinical trials. Therefore, patient centricity should be at the heart of every clinical trial, with the introduction of an array of technologies and services to enhance patient engagement.

The Perceptions & Insights study (2019) revealed a growing prevalence of solutions geared towards enhancing convenience. The adoption of smartphone applications and wearable devices garnered significant positive feedback. This underscores the importance of incorporating various convenience-enhancing approaches into clinical trial designs. This approach allows patient participants to select options that align with their individual needs, taking into account factors like work commitments, social media routines, childcare responsibilities, and the involvement of caregivers.
Navitas Life Sciences remains committed to improving health care by supporting efficient clinical trials, with patient centricity as the driving force.
Learn more about how we can support your decentralized clinical trials by leveraging technology & analytics.
To know more about our services and solutions, reach out to us at