Solutions
Advisory Services
Clinical Development
- Generics Development
- Clinical Operations
- Clinical Data Sciences
- Medical and Safety Services
- RWD & RWE Services
Post Marketing
- Safety Services
- Post marketing Studies
- Regulatory Affairs
By reimagining regulatory submission strategies with artificial intelligence in regulatory affairs, pharmaceutical companies can expedite timelines and boost their chances of successful product approvals.
To accelerate the market entry of new products, there is a need to increasingly focus on regulatory submissions as a critical area for developing strategic capabilities. The submission process is a multidisciplinary effort requiring meticulous coordination across phases to formulate a filing strategy and prepare the dossier for regulatory review.
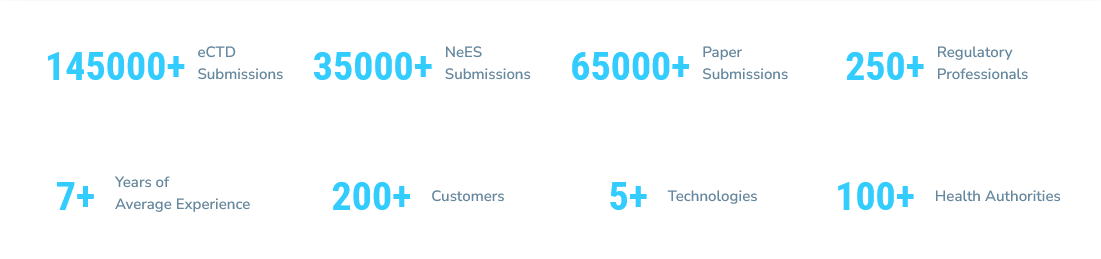
At Navitas Life Sciences, we define submission excellence as the ability to create high-quality regulatory submissions within industry-leading time frames. These submissions must accurately represent relevant data and maximize the likelihood of first-time approval with a favourable label for medical products. Achieving submission excellence benefits both patients and the pharmaceutical industry as faster product launches reduce waiting times for life-enhancing therapies.
A strategic partner with deep expertise on global regulatory affairs and a forward-thinking approach will aid in enhancing the regulatory submission process. Navitas Life Sciences provides a full spectrum of regulatory affairs services and regulatory affairs consulting designed to support pharmaceutical companies from development through post-marketing stages with regulatory time-to-market edge.
Our End-to-End Global Regulatory Affairs services include:
Ensuring patient safety and compliance with global pharmacovigilance regulations is paramount. Navitas Life Sciences offers robust regulatory and PV services, integrating artificial intelligence in pharmaceutical regulatory affairs to enhance efficiency and accuracy.
Our PV services include:


Marty Boom
Global Head of Regulatory & PV,
Navitas Life Sciences
Our Global Head of Reg. & PV, Marty Boom, is a seasoned expert with over two decades of experience in regulatory affairs, pharmacovigilance, and management consulting. Recently, he explored the targeted applications of Gen AI, and the use of artificial intelligence in pharmaceutical regulatory affairs in his enlightening article for PharmaFocus Europe.
We had the opportunity to sit down with Marty Boom to discuss the transformative impact of Generative artificial intelligence(AI) in regulatory affairs and pharmacovigilance.
How has Generative AI impacted pharmacovigilance and regulatory affairs in the life sciences industry?
Generative AI has significantly optimized workflows in pharmacovigilance and regulatory affairs, allowing for the automation of routine tasks and enhancing data analysis capabilities. This has led to increased efficiency, accuracy, and faster decision-making, ultimately improving regulatory compliance and patient safety.
Please share insights into how Generative AI has transformed adverse event reporting and improved patient safety or reporting accuracy?
Generative AI in regulatory affairs has revolutionized adverse event reporting by enabling automated case creation from unstructured data, improving the generation of case narratives, and facilitating prompt follow-ups. These advancements ensure more comprehensive and timely reporting, which enhances patient safety and reporting accuracy.
What emerging trends or innovations in Generative AI do you believe will further revolutionize PV and regulatory affairs?
Emerging trends in Generative AI that will further revolutionize PV and regulatory affairs include the use of AI for early detection of safety signals, the development of nearly touchless PV case processing, and the auto-generation of regulatory reports and narratives. These innovations will lead to more proactive safety management and streamlined regulatory processes.
Gain valuable insights into how AI in regulatory affairs is reshaping the life sciences industry

Explore transformative potential of Generative AI in pharmacovigilance and regulatory affairs.
Navitas Life Sciences is committed to driving innovation and excellence across phases, including in regulatory affairs and pharmacovigilance. Partner with us to leverage the power of Generative AI and transform your regulatory and safety processes for superior outcomes.
Contact us today to learn more about our regulatory affairs services and how our regulatory affairs consultants can support your journey towards faster market reach.
To learn more about our services and solutions, reach out to us at